Molecules That Curb Errant Proteins of AL Amyloidosis Point to New Type of Therapy
Scientists at Scripps Research have identified a group of small molecules that prevent structural changes to proteins that are at the root of AL amyloidosis, a progressive and often fatal disease.
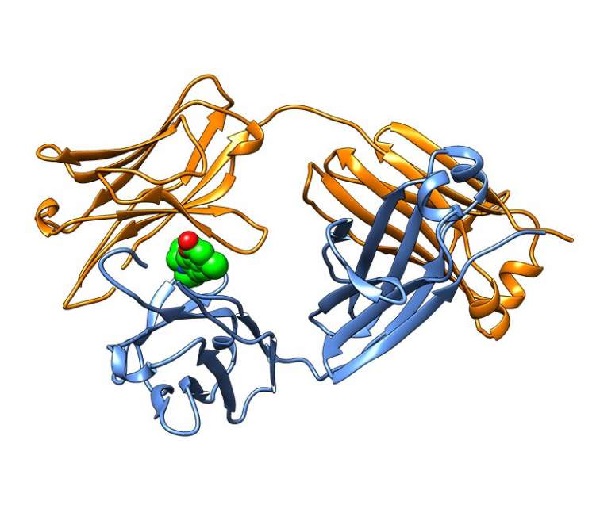
The small molecules bind to immunoglobulin proteins, which play an essential role in the body’s immune system, and then stabilize subunits of the protein called immunoglobulin light chains. Binding and stabilizing the light chains in their native shape prevents them from misfolding and forming the toxic plaques found in patients with AL amyloidosis.
By labeling light chains with fluorophores and coupling shape changes to cleavage by proteinase K, nearly a million small molecules were screened for their ability to prevent the disease-associated structural changes using fluorescence polarization. Using this strategy as the basis for a high-throughput screen and various distinct counter-screens to eliminate artifacts, the team discovered multiple small-molecule drug candidates that prevented immunoglobulin light chains from misfolding and aggregating in a test tube.

